We are pleased to announce that Skinive has received CE Mark clearance in the European Union.
The CE marking confirms that Skinive solutions meet the requirements of the European Medical Devices Directive (MDD). This clearance opens up the market significantly for our solutions. The CE mark covers 27 countries in the European Union. More importantly it validates our AI-powered solution as credible option and gives physicians and patients within the EU access to a reliable tool so they can identify skin conditions in an affordable and accessible manner.
As we stand today, We are proud to say we have achieved this.
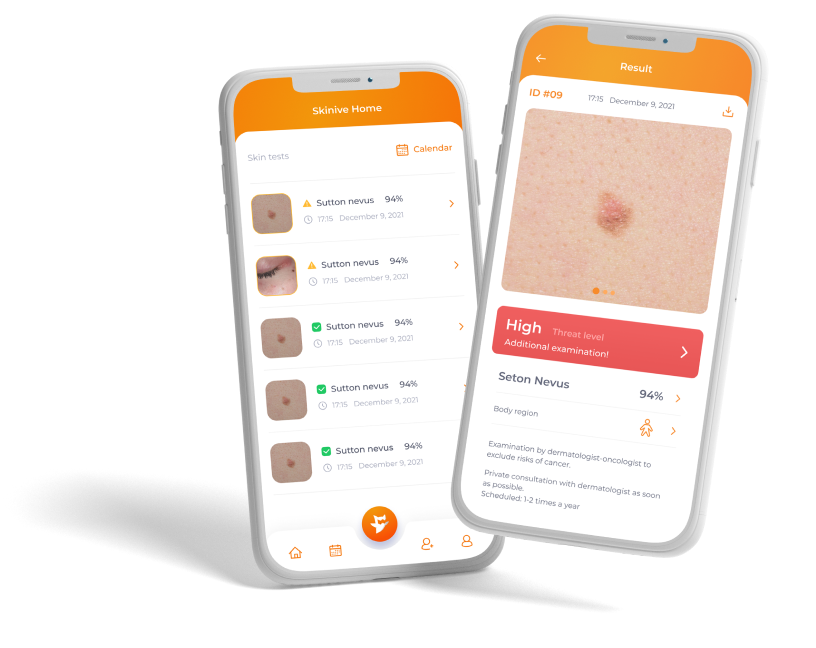
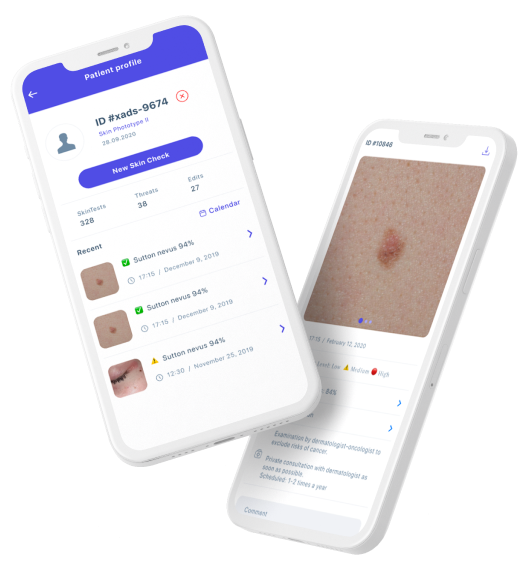
Skinive offers disruptive, comprehensive deep-tech diagnostic and monitoring solutions for home and professional use.
We possess an innovative product, based on AI and our own database.
The tool for early-stage diagnostics and monitoring of skin pathologies available for everyone.
“Receiving CE mark reinforces our mission to urgently deliver our technology to patients around the world and we are excited to get started” Kirill Sokolov, CEO of Skinive Holding.
The device will aid dermatologists, doctors and nurses in obtaining a quick second opinion and improve detection of dermatologic conditions.
It will also bring great value to patients as receiving care in the comfort of their own homes.